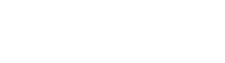More news
- Focus on industrial: Powering the energy industry during extreme heat
- Focus on powder coatings: The coatings industry’s transition to PFAS/PTFE-free solut...
- “We see sustainability as a purpose, as a reason for doing business” – P...
- Focus on industrial: High-performance coating protects tanks at biopolymer production plan...
- Focus on powder coatings: Novel high-speed crosslinking technology

Biofouling is a major concern in marine industries. The use of traditional toxic antifouling coatings is forbidden or severely restricted. This study aimed to provide a green and effective antifouling coating
Authors: Zhenze Liu (1), Yicong Zhang (2), Tiny Wang (3), Wenbo Du (4) and Huichao Jin (2)
Biofouling of marine vessels can increase the surface roughness, leading to increased drag resistance and fuel consumption. Biofouling accelerates surface corrosion and affects equipment safety; therefore, it is a major issue in the marine industry. Traditional antifouling coatings contain toxic chemicals such as mercury, arsenic and tributyltin (TBT). Although these toxic coatings are effective in combating biofouling, they exhibit teratogenic and lethal effects on other marine organisms. Their most serious consequence is that they can harm human health by bioaccumulating in food chains. Several countries have reported the presence of TBT in humans. In 2001, the International Maritime Organization (IMO) passed a ban on the application of TBT by 1 January 2003, and a total ban on the use of TBT by 1 January 2008. Therefore, it is necessary to develop environmentally friendly and effective antifouling coatings.
Biofouling is a stepwise process that involves the formation of a conditioning film, a biofilm and macroscopic fouling. Preventing biofilm formation (where the major contributors are bacteria and diatoms) can inhibit the subsequent biofouling stages. Recently, several metal ions, including zinc (Zn2+), copper (Cu+ and Cu2+), and silver (Ag+) ions, have been used to combat biofouling because of their microbial activity. The toxicity of these metal ions (e.g., Cu) is lower than that of TBT, and they are considered relatively green antifouling biocides under government supervision and approval.
Pristine or modified metal ions are mostly used as fillers to enhance the antifouling capabilities of coatings. For example, silicone/ZnO nanorod composite coating, ZnO/multi-walled carbon nanotube/polyethersulfone composite coating, polyhexanide-coated copper oxide nano-particles/poly (vinylidene fluoride) composite coating, cuprous oxide microcapsule/polyvinylpyrrolidone antifouling coating, AgNPs/poly(di(ethylene glycol)methyl ether methacrylate) coating, Ag@TA-SiO2 nanospheres/terpolymer coating, and Ag/ polydimethylsiloxane (PDMS) composite coating have shown potential for preventing marine biofouling.
However, most of these solutions involved a single metallic ion. A recent study showed that a single metal-based coating has antifouling limitations (e.g., limited broad-spectrum antifouling capability and durability) under various conditions. Therefore, combining metal ions with other materials to fabricate synergistic antifouling coatings has attracted the attention of researchers. Tian et al. reported a Cu-Ti composite coating by plasma spraying of mechanically mixed Cu/Ti powders, and the coating showed remarkable antifouling efficiency against bacterial survival and adhesion up to ∼100%. Calabrese et al. investigated the synergistic effects of Cu, Ag and titanium dioxide (TiO2). The Cu/TiO2 powder exhibited the best antifouling performance due to its synergistic effect. Recently, many metallic-ion combinations, including ZnO/Fe2O3, graphene/Ag, g-C3N4/ZnO, and Cu/TiO2 have exhibited enhanced antifouling capabilities.
To the best of our knowledge, no previous studies have investigated the antifouling capabilities of the Zn2+/Cu+combinations. Cu-doped zinc sulfide (ZnS:Cu) is a semiconductor material that emits luminescence under mechanical forces. In this study, ZnS:Cu was incorporated into polydimethylsiloxane (PDMS) as a filler to investigate the synergistic antifouling effects of Zn2+ and Cu+. PDMS is an elastic material that can undergo deformation under the impact of water flow; thus, the ZnS:Cu/PDMS composite coating can emit luminescence in flowing water. Several studies have revealed that luminescence can inhibit algal settlement; hence, the ZnS:Cu/PDMS composite coating has double antifouling effects, that is, microbial activity (Zn2+/Cu+) and luminescent drive effects. In the antifouling tests, Bacillus Subtilis (B. Subtilis) and Chlorella were selected as fouling models to evaluate the antifouling capabilities of the ZnS:Cu/PDMS composite coatings.
READ MORE:
Focus on marine: Hempel dives into sustainability collaboration
Preparation of ZnS:Cu/PDMS Coating
Method 1: First, 0.25g of ZnS:Cu, 25 g of PDMS, and 2.5g of the curing agent were mixed for 5min using a mechanical stirrer at 80rpm. The mixture was then placed in a vacuum oven at room temperature to remove internal bubbles. When no bubbles overflowed, the mixture was poured into an acrylic model (groove depth = 1 mm). After 48hr of curing at room temperature, a 1 wt% ZnS:Cu/PDMS coating was obtained.
Method 2: Because micro- and nano-particles have a high specific surface per unit volume, they exhibit high surface free energies (SFEs). It is difficult to distribute these particles uniformly in a low-surface-energy polymer matrix (e.g., silicone and fluorine polymers) due to the differences in their SFEs. Additionally, micro- and nano-particles are affected by gravity. Hence, during curing, the particles tend to move toward the bottom of the composite material. Therefore, the ZnS:Cu/PDMS coating prepared using Method 1 may have limitations in terms of dispersibility. A new preparation method was used to address this issue as follows:
First, 25g of PDMS and 2.5g of the curing agent were mixed for min using a mechanical stirrer at 80rpm, and the mixture was placed in a vacuum oven at room temperature to remove the internal bubbles. The mixture was allowed to stand in air for A hours to increase its viscosity, after which 0.25g of ZnS:Cu was added and stirred for 30min. The ZnS:Cu/PDMS mixture was poured into an aluminium alloy model (groove depth = 1mm) and placed in a vacuum oven to remove the internal bubbles. The model was then placed on a heating platform under B °C for C mins. After curing, a 1 wt% ZnS:Cu/PDMS coating was prepared. In addition, 10, 20, and 50wt% ZnS:Cu/PDMS samples were prepared, and pristine PDMS (0wt%) was used as the control group. Based on our experience, the viscosity after 5–8hr was accepted for preparation. According to the technical data sheet of SYLGARD 184 Silicone Elastomer, the curing time of PDMS at 25, 100, 125, and 150°C are 28hr, 35min, 20min, and 10min, respectively. Considering the effects of incorporated ZnS:Cu particles and coating thickness, the curing parameters of 80°C (240min) and 150°C (20min) were used.
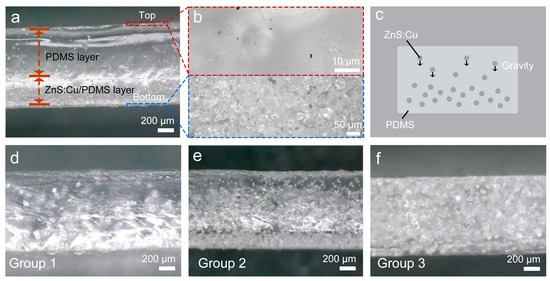
where θ is the WCA and γS and γL are the SFEs of solid and liquid, respectively. β can be considered a constant with the value of 1.129 × 10−4 m2/mJ. The γL of DI water is 72.8 mJ/m2. Surface chemical state information was analysed using an X-ray photoelectron spectrometer (XPS, ESCALAB 250XI, ThermoFisher Scientific, Waltham, MA, USA) with Al Ka x-rays. The chemical groups of the samples were analysed using a Fourier-transform infrared (FT-IR) spectrometer (Nicolet iS10, Thermo Scientific, Waltham, MA, USA). The ZnS:Cu sample for the FT-IR measurements was prepared using the KBr pellet method, and the thickness of the prepared sample was tens of microns. The surface elements were measured using an energy-dispersive spectrometer (EDS, HORIBA EMAX, HORIBA, Ltd., Kyoto, Japan). Tensile tests were performed using a tensile tester (UTM5305, YOUHONG, Shanghai, China), and the reference measurement procedure was GB/T 528-2009. To test the weight loss of the samples in a water environment, the pristine samples were immersed in DI water for 72hr, after which the samples were placed into a vacuum oven at 40°C for 48hr. After drying, the weight loss of each sample was calculated as follows: where M0 is the weight of the pristine sample and M1 is the weight of the dried sample after immersion. where A and B are the numbers of bacterial colonies in the control and experimental samples, respectively. where g is the acceleration due to gravity; ρs and ρf are the densities of particles and fluid, respectively; μ is the fluid viscosity; and D is the particle diameter. The settling distance is derived as follows:
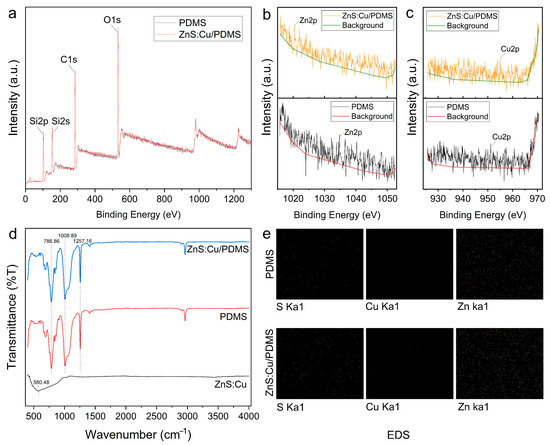
where t denotes the settling time. Two strategies are available for reducing the settlement of the ZnS:Cu particles in the PDMS: reducing the settling velocity (V) and reducing the curing time (t). According to Equation (4), V can be decreased by decreasing μ. During the curing process, the viscosity of PDMS increased with increasing time and temperature. Hence, the PDMS/curing agent mixture was allowed to stand in air for several hours to increase the viscosity of the PDMS. Another essential condition is the curing time; particle settlement in the PDMS stops when the PDMS is cured. Therefore, the ZnS:Cu particles in Group 3 (Figure 1f) showed the best dispersibility among the samples. Because particle dispersibility can affect the performance of the coating, the parameter in Group 3 was a good choice for preparing such coatings. Figure 3b shows the results of the tensile tests. The results indicate that the stress–strain curve decreased with increasing ZnS:Cu content. This may have been caused by stress concentration, and the ZnS:Cu particles in the PDMS provided the stress concentration points.
However, the stress–strain curve of the 50wt% sample did not follow the aforementioned trend. This is because excessive ZnS:Cu particles in the PDMS provide sufficient tensile strength, and the effect of the stress concentration becomes weak. We assume that all the samples are subjected to a tensile force of 1 MPa, and a higher strain indicates that the coating is prone to deformation, that is, softer. According to fracture mechanics and tests, the elastic modulus and stiffness of coatings influence the adhesion of fouling organisms. Specifically, a low elastic modulus and soft coating are helpful in preventing biofouling adhesion. Hence, the incorporation of ZnS:Cu into PDMS helps combat biofouling.
Figure 3c shows that the weight loss in the 0 wt% sample is 0.13 ± 0.1%, indicating that the pristine PDMS can lose weight in the test procedure. When ZnS:Cu was incorporated into the PDMS, the weight loss in all composite samples increased. This indicates that the ZnS:Cu particles can be released into water. The weight loss in the ZnS:Cu/PDMS coatings decreased with increasing ZnS:Cu content. A possible reason is that the increased ZnS:Cu ratio resulted in enhanced compactness of the composite coatings; thus, the channel for water molecules to enter the composite coatings was narrowed. However, the weight loss in the 50wt% sample was greater than that of the 20wt% sample. As excess ZnS:Cu particles were incorporated into the 50wt% sample, the number of ZnS:Cu particles exposed to water molecules increased.
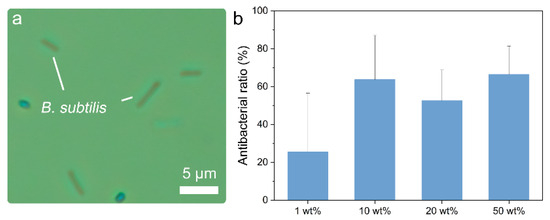
Because bacteria and algae are the major contributors to the primary stage of biofouling, antifouling tests against B. subtilis and Chlorella were performed. B. subtilis is commonly found in marine environments. It is a typical rod-shaped bacteria with a length of 2–4μm (Figure 4a). In the antifouling test against B. subtilis, the 1wt% sample showed an average antibacterial ratio of 25.5% (Figure 4b). The antibacterial ratio increased to 63.8% when the ZnS:Cu content increased from 1 to 10 wt%. However, when the ZnS:Cu content was increased to 20wt%, the antibacterial ratio decreased to 52.6%. A possible reason for this is that the ZnS:Cu release rate of the 10 wt% sample was higher than that of the 20 wt% sample (Figure 3c), but the latter was softer than the former (Figure 3b); hence, both effects resulted in the 20wt% sample exhibiting reduced antifouling capability. The 50wt% sample had the highest ZnS:Cu content and exhibited the highest antibacterial ratio of 66.4%, indicating its excellent antifouling capability. The possible antibacterial mechanisms of ZnS:Cu can be analysed based on the antibacterial activities of pristine ZnS and Cu.
According to previous reports, the generating of biologically reactive oxygen species (ROS) is the antibacterial mechanism of ZnS. These ROS, such as superoxide anions, hydroxyl ions, and hydroxyl radicals, damage bacterial cells by attacking cytoplasmic and extra-cytoplasmic targets. The Cu ions have multiple antibacterial effects, including ROS generating, protein oxidation, DNA degradation, and lipid peroxidation.
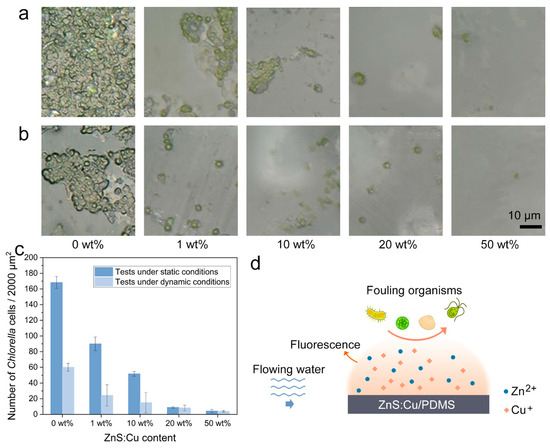
The antifouling tests against Chlorella were conducted under static and dynamic conditions. In the static tests (Figure 5a), many Chlorella cells covered the 0wt% sample. Among the samples with ZnS:Cu contents, the 1wt% and 10wt% samples showed several Chlorella colonies, whereas the 20wt% and 50wt% samples showed only a few scattered Chlorella cells. According to the quantized data in Figure 5c, the antifouling capability of the samples increased with increasing ZnS:Cu content.
This variation trend was different from that observed in the antibacterial tests, indicating that the ZnS:Cu particles have a selective antifouling capability. In the dynamic tests, the antifouling capability of the samples increased with increasing ZnS:Cu content. The numbers of Chlorella cells on the 0 wt%, 1 wt%, and 10 wt% samples were significantly decreased in the dynamic tests (Figure 5b) compared with those in the static tests (Figure 5a), and the numbers of Chlorella cells on the 20wt% and 50wt% samples showed a reduction to some extent. There are several mechanisms underlying these results in the dynamic tests. First, ZnS:Cu particles may have a high release ratio in flowing water. Second, deformation is more likely to occur on the ZnS:Cu/PDMS surface than on the pristine PDMS under the impact of water flow; thus, fouling organisms can easily detach from the surface during continuous deformation.
Thirdly, surface deformation may induce fluorescence to prevent the settlement of Chlorella. Hence, the samples exhibited better antifouling capabilities under dynamic conditions than under static conditions. A schematic illustration of the antifouling mechanisms of the ZnS:Cu/PDMS coatings is shown in Figure 5d. According to these tests, the 50wt% sample exhibited the best antifouling capability. Although coatings with a higher ZnS:Cu content (e.g., 60wt%) may have better antifouling capabilities, this can lead to a significant increase in costs. Hence, the 50wt% sample is a good candidate for practical applications.
A series of ZnS:Cu/PDMS coatings with different ZnS:Cu contents was prepared. The preparation method was simple and cost-effective. These coatings exhibited hydrophobicity, which is conducive to combating biofouling. The ZnS:Cu/PDMS coatings exhibited excellent antifouling capabilities against B. subtilis and Chlorella.Materials and methods
Materials
Characterisation analysis
cos 𝜃=−1+2𝛾S𝛾L−−−√ [1–𝛽 (𝛾L−𝛾S)2]
Antifouling tests
Results and discussion
Preparation optimisation
Characterisation analysis
READ MORE:
Antifouling tests
Conclusions